 The Covid-19 vaccine is not safe and should it cause permanent damage to health, or even kill, the UK Government have made sure that the vaccine manufacturer will not be made liable for damages. Anyone with a right mind should realise that this vaccine is experimental, as no other vaccine has been made in such a short space of eight months. Matt Hancock rejoices and weeps at the ‘good news’, as do other MPs, but these brainwashed politicians should be weeping in terror knowing that this vaccine will cause a high number of adverse drug reactions. The medicines authority expects it.
The Covid-19 vaccine is not safe and should it cause permanent damage to health, or even kill, the UK Government have made sure that the vaccine manufacturer will not be made liable for damages. Anyone with a right mind should realise that this vaccine is experimental, as no other vaccine has been made in such a short space of eight months. Matt Hancock rejoices and weeps at the ‘good news’, as do other MPs, but these brainwashed politicians should be weeping in terror knowing that this vaccine will cause a high number of adverse drug reactions. The medicines authority expects it.
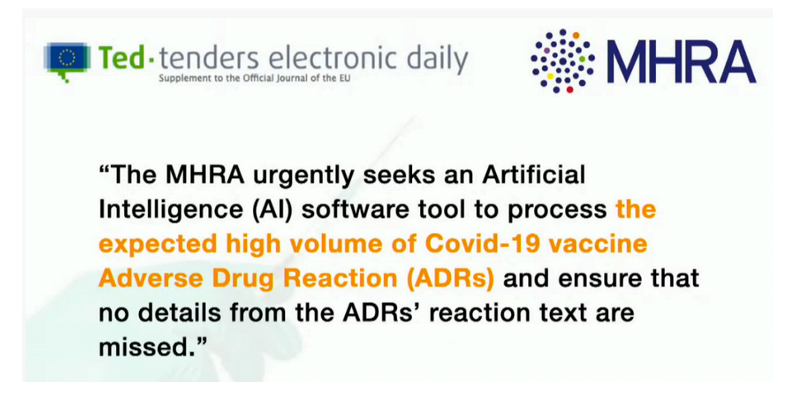
The Medicines and Healthcare Products Regulatory Authority (MHRA) have put an advertisement out requesting an artificial intelligence software tool to process the expected high volume of Covid-19 vaccine adverse drug reactions (ADRs). Make no doubt about it, these vaccinations are part of the depopulation agenda and there to profit the globalists who do not care about human health, just like our UK Government.
This is not conspiracy, to those who want to deny such a fact! Here is the actual PDF.
MHRA URGENTLY SEEKS ARTIFICIAL INTELLIGENCE SOFTWARE TOOL TO PROCESS THE EXPECTED HIGH VOLUME OF COVID-19 VACCINE ADVERSE DRUG REACTION (ADRS)
I encourage you to watch this video where Dr Andy Wakefield gives warning about the Adverse Drug Reactions that the Covid-19 vaccine may cause to millions of people, and he estimates that the Covid-19 vaccine has a six month timespan before it will have proven its ineffectiveness in causing permanent adverse reactions, or even death, to its victims.
Adverse drug reactions (ADRs): Summary – NICE
- An adverse drug reaction (ADR) is an unwanted or harmful reaction which occurs after administration of a drug or drugs and is suspected or known to be due to the drug(s).
- ADRs have traditionally been categorized as Type A and Type B:
- Type A reactions (pharmacological/augmented) result from an exaggeration of a drug’s normal pharmacological actions when given at the usual therapeutic dose. They are dose dependent and are therefore readily reversible on reducing the dose of (or withdrawing treatment with) the drug.
- Type B reactions (idiosyncratic/bizarre) cannot be predicted from the known pharmacology of the drug.
- Type A adverse reactions are more common than Type B reactions and account for more than 80% of all reactions.
- The health and financial implications of ADRs are significant: about 6–7% of hospital admissions in the UK are due to ADRs.
- The Medicines and Healthcare products Regulatory Agency (MHRA) and the Commission on Human Medicines (CHM) are responsible for monitoring the safety of all medicines marketed in the UK, through the process of pharmacovigilance.
- Spontaneous ADR reporting schemes (such as the Yellow Card Scheme) are important information sources for pharmacovigilance.
- Data from the Yellow Card Scheme underpins the process of pharmacovigilance in the UK.
- Yellow Card reports should be made for all suspected ADRs in adults and children that are:
- Serious, medically significant, or result in harm.
- Associated with Black Triangle products, including suspected ADRs considered not to be serious.
- Yellow Card reports can be made for:
- Suspected ADRs to all medicines, including vaccines, blood factors and immunoglobulins, and complementary medicines.
- All medical devices available on the UK market.
- Defective medicines (those that are not of an acceptable quality).
- Fake or counterfeit medicines or medical devices.
- Nicotine-containing electronic cigarettes and refill containers (e-liquids).
- The MHRA are particularly interested in receiving Yellow Card reports of suspected ADRs:
- In children.
- In people aged over 65 years.
- To biological medicines and vaccines.
- Associated with delayed drug effects and interactions.
- On congenital abnormalities.
- To complementary remedies.
- It is not necessary to be certain that an ADR has occurred before submitting a report.
- A Yellow Card can be submitted:
- Via the MHRA Yellow Card website (www.mhra.gov.uk/yellowcard).
- Via a free Yellow Card mobile app.
- By post. Paper copies of the Yellow Card can be obtained from several sources, for example by downloading the ‘Healthcare Professional Yellow Card Reporting Form’ from the MHRA website, by writing to FREEPOST YELLOW CARD, by emailing yellowcard@mhra.gsi.gov.uk, or by calling the Yellow Card Scheme helpline on 0808 100 3352 (10am to 2pm Monday–Friday).
- Members of the public can also report suspected ADRs (by using the Yellow Card Scheme) via the MHRA Yellow Card website, by telephone on 0808 100 3352, or by downloading the ‘Member Of Public Yellow Card Reporting Form’ from the MHRA website. Patient Yellow Cards are also available from pharmacies and GP surgeries.

This makes me wonder if it’s time to move to Texas.
Everywhere is chaotic. The only safe place is in the hands of Jesus. The gospel: https://youtu.be/StgAtwxiix0